Time is prognosis in heart failure: Significant benefit with early initiation of SGLT2 inhibitors

Growing evidence supports early initiation of guidelinedirected medical therapy (GDMT), including sodium-glucose cotransporter 2 (SGLT2) inhibitors, in patients with heart failure (HF). At a Boehringer Ingelheim–sponsored symposium in Hong Kong, Professor Piotr Ponikowski of the Medical University, Wrocław, Poland, discussed how this approach may improve patients’ prognosis, highlighting randomized controlled trial (RCT) evidence supporting in-hospital initiation of empagliflozin in acute HF and sharing a case study to illustrate practical aspects of this approach.
Why in-hospital initiation of GDMT for HF?
“Increasing evidence highlights the importance of early initiation of GDMT
for HF, including SGLT2 inhibitors, in the hospital setting as soon as clinical stability
is achieved, followed by well-planned transition to chronic care,” said Ponikowski. “Hospitalization for HF [HHF] provides an ideal window of opportunity to initiate and titrate GDMT in a rapid and concurrent manner and to plan nonpharmacological interventions.” [Eur Heart J Suppl 2022;24:L38-L44]
This new paradigm, supported by results of clinical trials including STRONG-HF and EMPULSE, can mitigate the risk of poor outcomes during the vulnerable postdischarge phase. [Lancet 2022;400:1938-1952; Nat Med 2022;28:568-574; Eur Heart J Suppl 2022;24(Suppl L):L38-L44]
In the STRONG-HF trial in 1,078 patients hospitalized for acute HF (mean age, 63 years; female, 39 percent), predischarge initiation of GDMT followed by rapid uptitration to full recommended doses within 2 weeks of discharge, along with close follow-up during the first 2 months postdischarge, was associated with a significantly reduced risk of 180-day HF readmission or all-cause mortality (primary endpoint) vs usual care (15.2 percent vs 23.3 percent; risk ratio, 0.66; 95 percent confidence interval [CI], 0.50–0.86). [Lancet 2022;400:1938-1952]
Empagliflozin’s benefit in acute HF
“In the EMPULSE trial, in-hospital initiation of empagliflozin in patients with stabilized
acute HF provided significant clinical benefit vs placebo,” said Ponikowski. [Nat Med 2022;28:568-574]
In-hospital initiation after clinical stabilization
The trial included 530 patients (median age, 71 years; female, 34 percent) hospitalized
for acute de novo or decompensated chronic HF regardless of left ventricular
ejection fraction (LVEF). The patients were randomized 1:1 in hospital, when clinically
stable (median time from admission to randomization,
3 days), to receive empagliflozin 10 mg QD or placebo for ≤90 days. The
primary outcome of clinical benefit, defined as a hierarchical composite of all-cause mortality,
number of HF events and time to first HF event, or a ≥5-point difference in change
from baseline in Kansas City Cardiomyopathy Questionnaire Total Symptom Score at
90 days, was assessed using a win ratio.
Significant clinical benefit
“Overall clinical benefit was 36 percent more likely with empagliflozin vs placebo [stratified win ratio, 1.36; 95 percent CI, 1.09–1.68; p=0.0054]. Importantly, empagliflozin’s clinical benefit was consistent regardless of HF status [ie, de novo or decompensated chronic HF] or baseline LVEF,” said Ponikowski. (Figure)
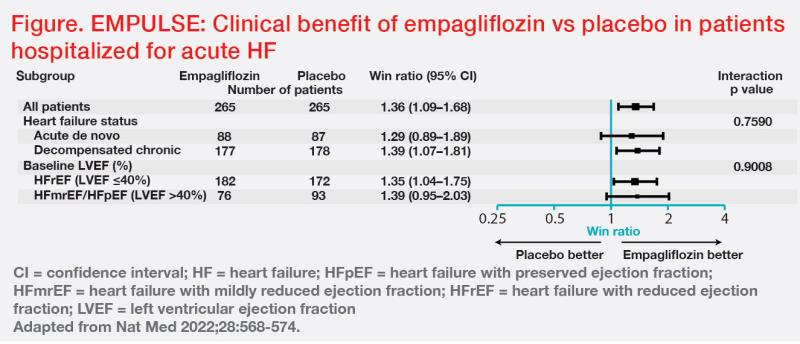
An exploratory analysis showed a 35 percent relative risk reduction in HF events or all-cause mortality with empagliflozin vs placebo (14 percent vs 22 percent; hazard ratio, 0.65; 95 percent CI, 0.43–0.99; p=0.042), with a number needed to treat of 15. [Teerlink J, et al, HFSA 2022] Markers of decongestion also showed significantly greater reductions with empagliflozin vs placebo. [Eur Heart J 2023;44:41-50]
“Rates of adverse events were similar between the empagliflozin and placebo arms, with no safety signals observed. No ketoacidosis was reported,” said Ponikowski. [Nat Med 2022;28:568-574]

Related MIMS Drugs





 Change Password
Change Password
 Points
Points
 Sign Out
Sign Out

