Dermatology

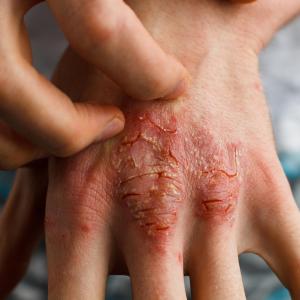


Once-daily treatment with topical roflumilast cream 0.15% demonstrates a significant improvement in vIGA-AD* score, achieving a clear or almost clear status, in patients with mild-to-moderate atopic dermatitis (AD), according to the pooled analysis of the phase III INTEGUMENT-1/2 studies presented at ACAAI 2023.



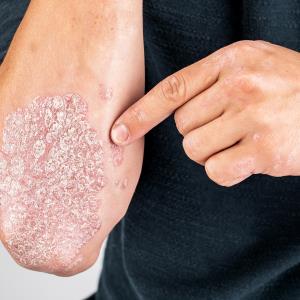
The patient is a 29-year-old male who was first diagnosed with psoriatic arthritis (PsA) in November 2010. He had comorbid well-controlled epilepsy and obesity, with body weight fluctuating between 100 kg and 107 kg in recent years.


Once-daily treatment with topical roflumilast cream 0.15% demonstrates a significant improvement in vIGA-AD* score, achieving a clear or almost clear status, in patients with mild-to-moderate atopic dermatitis (AD), according to the pooled analysis of the phase III INTEGUMENT-1/2 studies presented at ACAAI 2023.

Subcutaneous prophylactic treatment with garadacimab significantly reduced the monthly number of attacks and led to attack-free status as early as week 1 in patients with hereditary angioedema (HAE) in the post hoc analysis of the phase III VANGUARD study presented at ACAAI 2023.






 Change Password
Change Password
 Points
Points
 Sign Out
Sign Out