The future of COVID-19 management: A spotlight on respirology
At the 27th Congress of the Asian Pacific Society of Respirology (APSR) 2023, a Pfizer-sponsored symposium addressed the evolving challenges of COVID-19, particularly for patients with pre-existing respiratory conditions. Chaired by Dr Chan Kwok Wai Adrian, a renowned respiratory physician and intensivist from Mount Elizabeth Novena Hospital, Singapore, and featuring insights from Professor Yang Kuang-Yao of National Yang Ming Chiao Tung University, Taipei, Taiwan, the symposium focused on the disease’s impact, treatment guidelines, regional management strategies, and the role of nirmatrelvir/ritonavir, a new oral combination pill, in the treatment of COVID-19.
COVID-19 remains a serious health threat in Asia
“COVID-19 persists as a grave menace with far-reaching consequences for society. In Southeast Asia alone, there have been over 61 million reported cases of COVID-19, leading to a staggering toll of over 800,000 deaths. [World Health Organization, https://www.who.int/southeastasia/outbreaks-and-emergencies/covid-19/what-is-happening/sear-weekly-situation-reports] The pandemic has thrust approximately 80 million people in Asia into extreme poverty. [World Economic Forum, https://www.weforum.org/agenda/2021/08/covid19-pandemic-asia-pacific-progress-global-development-goals] “It is indeed the most drastic public health emergency the world has encountered in the past century,” Yang said.
In Singapore, the age-standardized death rate increased from 525 in 2019 to 549.9 per 100,000 person-years during the pandemic, translating to an estimated 2,490 excess deaths over the 2.5-year period. [Singapore MOH, Public report on excess mortality during the COVID-19 pandemic, https://www.moh.gov.sg/news-highlights/details/public-report-on-excess-mortality-during-the-covid-19-pandemic]
Risk factors for severe COVID
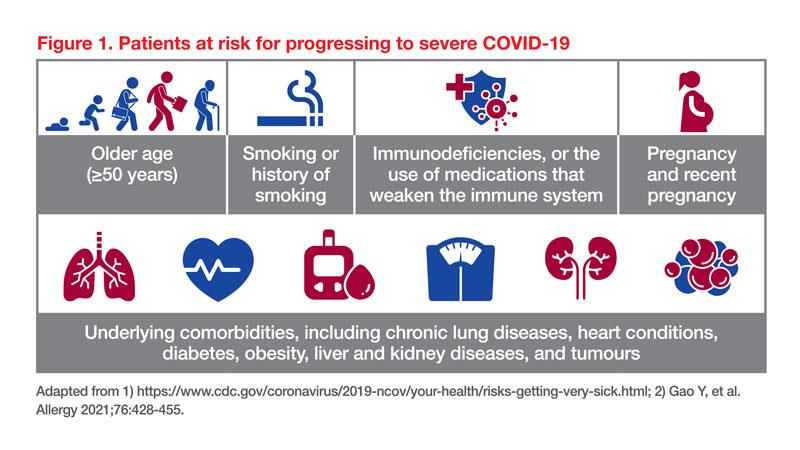
Several risk factors contribute to the progression of COVID-19 to a severe and critical stage (Figure 1). Yang particularly highlighted the association between pre-existing respiratory conditions and severe COVID-19. A UK population cohort study showed that patients with chronic obstructive pulmonary disorder (COPD), other interstitial lung disease (ILD), and asthma had an increased risk of hospitalization due to severe COVID-19, with hazard ratios (HR) of 1.54, 1.66, and 1.18, respectively. [Lancet Respir Med 2021;9:909-923]
Smoking has also been established as a risk factor for severe COVID-19. According to a meta-analysis, current and former smokers have a 30–50 percent excess risk of COVID-19 progression compared with never-smokers, a 16 percent higher risk of being hospitalized and a 39 percent higher risk of COVID-19 mortality. [Eur Respir Rev 2023;32:220191] The impact of smoking on COVID-19 outcomes is primarily linked to smoking-related comorbidities such as cardiovascular disease and COPD. Smoking-induced lung injury, changes in host defences, and compromised antiviral inflammatory cascades further contribute to underlying pathophysiology for COVID-19 severity. [Ann Am Thorac Soc 2021;18:1610-1613]
Clinical guidelines and recommendations
Yang presented an overview of the clinical guidelines for severity and management of COVID-19 (Figure 2).
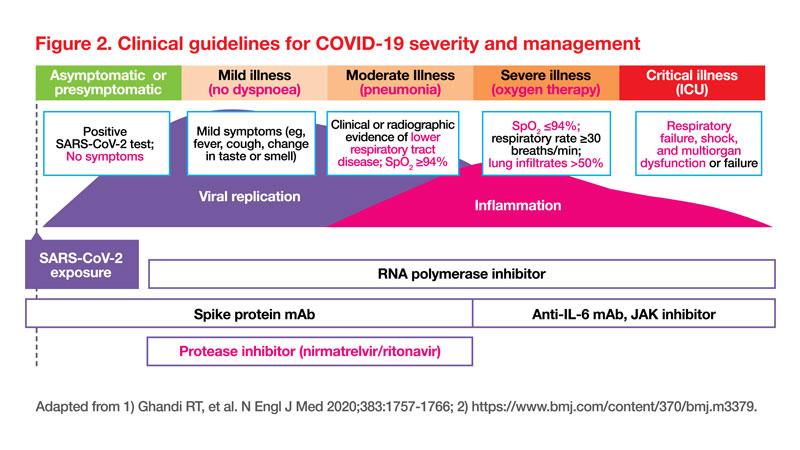
Various guidelines including the Centers for Disease Control and Prevention (CDC), the World Health Organization (WHO), and the National Institutes of Health (NIH) strongly recommend nirmatrelvir/ritonavir for patients with nonsevere COVID-19 who face a high or moderate risk of hospitalization. High-risk individuals are those who are immunosuppressed, while the moderate-risk category includes individuals older than 65 years old, or those with underlying comorbidities (Figure 1 for details). [CDC, https://www.cdc.gov/respiratory-viruses/whats-new/antiviral-treatments. html; NIH, COVID-19 Treatment Guidelines, https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/; WHO, WHO updates guidelines on treatments for COVID-19, Updated 10 Nov 2023, https://www.who.int/news/item/10-11-2023-who-updates-guidelines-on-treatments-for-covid-19#:~:tex-t=The%20recommendations%20state%20 that%20nirmatrelvir,by%20WHO%20in%20 April%202022]
“High-risk patients can quickly progress to severe disease, underscoring the need for rapid therapeutic intervention. [J Heart Lung Transplant 2020;39405-407] Nirmatrelvir/ritonavir treatment should be initiated as soon as possible after a diagnosis is made and within 5 days of symptom onset,” emphasized Yang. [CDC, https://www.cdc.gov/respiratory-viruses/whats-new/antiviral-treatments.html]
Clinical trials on nirmatrelvir/ritonavir
The EPIC-HR trial was a randomized, placebo-controlled study that recruited more than 2,246 participants with mild-to-moderate COVID-19 and who had one or more risk factors for progression to severe COVID-19; 1,039 participants received nirmatrelvir/ritonavir and 1,046 received placebo. Nirmatrelvir/ritonavir reduced the risk of COVID-19-related hospitalization or death compared with placebo by 88.9 percent when treatment was given within 3 days of onset of symptoms and 87.8 percent when given within 5 days of onset of symptoms. [N Engl J Med 2022;386:1397-1408]
Nirmatrelvir/ritonavir may interact with various medications, and the potential for drug interactions should be carefully considered by the prescribing doctor prior to treatment initiation. Though co-medication administration may be complex, support is available.
Real-world evidence on nirmatrelvir/ritonavir
A retrospective cohort study of nonhospitalized COVID-19 patients in Hong Kong SAR examined the efficacy of molnupiravir and nirmatrelvir/ritonavir in reducing hospitalization and severe outcomes. After 30 days, nirmatrelvir/ritonavir users had a reduced hospitalization risk (hazard ratio [HR], 0.79), while molnupiravir users did not significantly differ from nonusers (HR, 1.17). This study suggests that the use of nirmatrelvir/ritonavir, but not molnupiravir, is associated with a reduced risk of hospitalization of COVID-19 patients in real-world settings. [Clin Infect Dis 2023;76:e26-e33]
In Singapore, a retrospective study involving over 140,000 adults aged ≥60 years found that nirmatrelvir/ritonavir reduced the risk of hospitalization during Omicron BA.2/4/5/XBB waves. Among the participants, most of whom were vaccinated with ≥3 doses of mRNA vaccines, those treated with nirmatrelvir/ritonavir had lower hospitalization odds (adjusted odds ratio, 0.65). In this highly vaccinated population, the drug did not significantly reduce the already low risk of severe COVID-19. [Clin Microbiol Infect 2023;29:1328-1333]
Similarly, a Malaysian study of 20,966 high-risk COVID-19 outpatients showed that treatment with nirmatrelvir/ritonavir for 5 days effectively reduced hospitalization risk by 36 percent compared with the untreated controls (n=10,483 in each arm). [Int J Infect Dis 2023:135:77-83]
“These independent studies consistently demonstrated that the use of nirmatrelvir/ritonavir reduced hospitalization rates among COVID-19 patients in real-world settings,” concluded Yang.






 Change Password
Change Password
 Points
Points
 Sign Out
Sign Out


