Role of CGRP-targeted therapies in acute and preventive management of migraine

Unmet needs remain in acute and preventive management of migraine despite availability of multiple treatment options in East Asia. At the 2023 International Headache Congress in Seoul, South Korea, Professor Peter Goadsby of King’s College London in London, UK, and Professor Min-Kyung Chu of Severance Hospital, Yonsei University in Seoul, South Korea, reviewed the role of calcitonin gene-related peptide (CGRP)–targeted therapies in migraine management, highlighting the efficacy and safety of the oral CGRP receptor antagonist (RA), rimegepant, in both acute treatment and prevention of migraine.
Unmet needs in migraine management
Migraine is a neurological disorder with different phases, including premonitory
phase (characterized by tiredness, yawning, concentration difficulties, neck
discomfort, and polyuria), pain (which may be associated with nausea, photophobia,
and phonophobia), and postdrome phase (characterized by tiredness
and poor concentration). [Brain 2014;137:232-241;
Nat Rev Dis Primers 2022;8:2]
“Globally, migraines are among the most common chronic and disabling neurological problems due to the characteristic recurrent attacks of headache and accompanying symptoms,” said Goadsby. [Lancet Neurol 2018;17:954-976; Nat Rev Dis Primers 2022;8:2]
“In East Asia, migraine is also associated with high levels of disability and negative effects on quality of life [QoL],”noted Chu. [Headache 2012;52:582-591; Neurol Ther 2022;11:205-222; J Headache Pain 2021;22:45] “In a 2019 survey in South Korea involving 207 patients with episodic or chronic migraine aged 15–76 years, high levels of disability [ie, mean Migraine Disability Assessment score, 48.4] and poor QoL [mean Migraine-Specific QoL Questionnaire version 2.1 score, 47.7] were reported,” he noted. “Importantly, only 40.8 percent and 27.1 percent of respondents reported treatment satisfaction with preventive and acute medications, respectively.” [J Headache Pain 2021;22:45]
Unmet needs in effective acute and preventive treatment of migraine were also exposed in the observational OVERCOME study (n=17,071) in Japan. Results showed that 87.1 percent of migraine sufferers who completed the survey were currently using acute treatments, while 42.0 percent reported poor effectiveness of treatment, 10.3 percent had contraindications to triptans, and 52.9 percent reported at least one cardiovascular risk factor, suggesting substantial opportunity of improving migraine care, including prescription of novel acute medications. [Adv Ther 2022;39:5176-5190]
Targeting CGRP in migraine management
CGRP is a pain-signalling neuropeptide expressed in trigeminal ganglia nerves, whose plasma levels increase during a migraine attack. It is a potent vasodilator of cerebral and dural vessels, which are important in regulating blood flow to the brain and painsensitive meninges. CGRP can also cause degranulation and subsequent release of inflammatory agents from meningeal mast cells and may be involved in transmission of painful stimuli from intracranial vessels to the central nervous system. Thus, blocking the pathophysiological activities of CGRP has emerged as a valid therapeutic target for acute and preventive treatment of migraine. [Nat Rev Neurol 2010;6:573-582;
N Engl J Med 2004;350:1073-1075; Nat Rev Neurol 2018;14:338-350]
Acute and preventive CGRP-targeted therapies include the CGRP RAs, gepants, and monoclonal antibodies (mAbs) against CGRP peptide or receptor. [Nat Rev Dis Primers 2022;8:2]
Rimegepant for acute and preventive migraine treatment
“Several gepants and anti-CGRP mAbs have been approved for either acute treatment or prevention of migraine,” noted Chu. “However, only Rimegepant is currently indicated for both acute and preventive treatment of migraine.” [Nat Rev Neurol 2018;14:338-350; Nurtec Hong Kong Prescribing Information, November 2022]
Acute treatment evidence
The efficacy and safety of oral rimegepant (75 mg) for acute migraine treatment were demonstrated in a double-blind, randomized, placebo-controlled, multicentre phase III trial that included adults aged ≥18 years with history of migraine of ≥1 year’s duration (n=1,375). Results showed that rimegepant was superior to placebo in the coprimary endpoints of freedom from pain (21 percent vs 11 percent; risk difference, 10; 95 percent confidence interval [CI], 6–14; p<0.0001) and freedom from the most bothersome symptom (35 percent vs 27 percent; risk difference, 8; 95 percent CI, 3–13; p=0.0009) at 2 hours post-dose. Tolerability was similar to placebo, with nausea (rimegepant, 2 percent; placebo, <1 percent) and urinary tract infection (rimegepant, 1 percent; placebo, 1 percent) as the most common adverse events (AEs). [Lancet 2019;394:737-745]
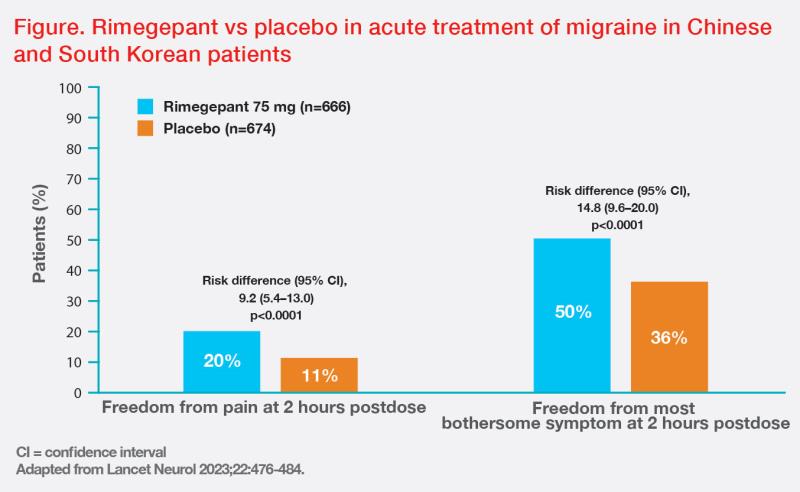
“Similar efficacy and safety results were reported in another phase III study in patients from China and South Korea,” said Chu. In this trial, rimegepant also demonstrated superiority vs placebo in the coprimary outcomes of freedom from pain and freedom from the most bothersome symptom at 2 hours postdose, as well as in all key secondary outcomes, including sustained freedom from pain 2–24 hours and 2–48 hours after dosing, pain relief 2 hours post-dose, rescue medication use within 24 hours after dosing, and normal functioning 2 hours after dosing. (Figure) [Lancet Neurol 2023;22:476-484]
Prophylaxis evidence
The efficacy and safety of Rimegepant for migraine prophylaxis were reported in a phase II/III, randomized, double-blind, placebo-controlled trial that included 747 adults with ≥1 year’s history of migraine, who received Rimegepant 75 mg or placebo every other day for 12 weeks after a 4-week observation period. Results showed superiority of rimegepant vs placebo in the primary endpoint of change from the observation period in mean number of migraine days per month during weeks 9–12 (-4.3 days vs -3.5 days; least squares mean difference, -0.8 days; 95 percent CI, -1.46 to -0.20; p=0.0099). Tolerability was also similar to placebo, with no unexpected or serious safety issues. [Lancet 2021;397:51-60]
Switching between CGRP-targeted therapies
“Since CGRP-targeted therapies have different formulations and sites of
action, switching between these novel therapeutics may widen treatment options
in difficult-to-treat patients, such as those with inadequate response to
previous therapies,” noted Chu. [Cephalalgia 2022;42:291-301;
Cephalalgia 2023;43:3331024221146315]
“Switching between CGRP-targeted therapies may also allow for dose escalations, different administration schedules [eg, daily, monthly, quarterly], and use of different formulations [eg, oral, intravenous or subcutaneous], although more studies are needed,” he added. [Cephalalgia 2023;43:3331024221146315]
Summary
CGRP-targeted therapies, including gepants and anti-CGRP mAbs, offer
specific, effective and well-tolerated options for acute or preventive treatment
of migraine. Rimegepant is currently the only CGRP-targeted therapy indicated
for both acute and prophylactic management of migraine, with proven efficacy
and tolerability in acute treatment of Asian patients.





 Change Password
Change Password
 Points
Points
 Sign Out
Sign Out


